Abstract
BACKGROUND
Diffuse large B cell lymphoma (DLBCL) is a heterogeneous entity with varied outcomes. A subset of DLBCL with high-risk features carries poor prognosis with an increased relapse rate when treated with chemoimmunotherapy regimen incorporating rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). Dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (DA.R-EPOCH) regimen is a high intensity regimen commonly used for the upfront treatment of high-risk DLBCL, especially DLBCL with c-Myc ± BCL2/BCL6 translocation. A phase 3 study (CALGB 50303) comparing R-CHOP and DA.R-EPOCH in untreated DLBCL did not show any difference in progression free survival (PFS) or overall survival (OS), however specific outcomes of high-risk DLBCL are unknown. (Wilson et al. ASH 2016) In this study, we analyzed the clinical outcomes in a longitudinal cohort of patients with high-risk DLBCL who received DA.R-EPOCH, and compared it to the similar group of patients who received R-CHOP during the same period at our institute.
METHOD
Patients diagnosed with DLBCL who received chemo-immunotherapy at our center between January 2011 to December 2016 were included in the analysis. High-risk DLBCL was defined by the presence of any of the following features at the time of diagnosis: International prognostic index (IPI) score >/= 4, tumor measuring >/=5 cm, c-Myc ± BCL2/BCL6 translocation by FISH or overexpression by immunohistochemistry, Ki-67 index >/= 80% and activated B-cell (ABC) phenotype by Han's algorithm. Patients without these features were deemed to have standard risk-DLBCL. Clinical characteristics, grade 3/4 toxicities (CTCAE v4) and need for hospitalization and transfusion support were reviewed. Responses were evaluated at middle and end of chemotherapy. Overall (OS) and progression free survival (PFS) were calculated. Cox proportional hazard regression was performed to assess influence of clinical and treatment variables on OS and PFS.
RESULTS
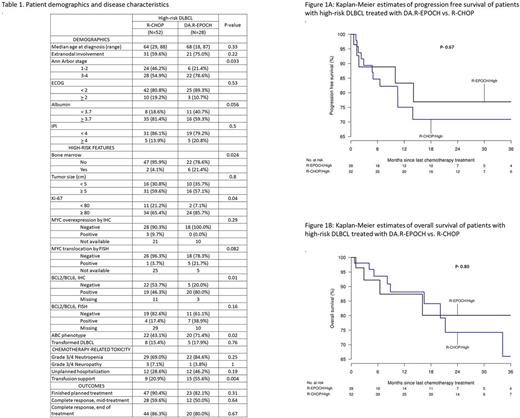
A longitudinal cohort of 95 patients was included in this analysis. All standard risk DLBCL (N=15) patients were treated with R-CHOP. Eighty patients with high-risk DLBCL were treated with R-CHOP (N= 52, 65%) and DA.R-EPOCH (N= 28, 35%) respectively. Table 1 shows baseline clinical characteristics of all high-risk DLBCL patients. DA.R-EPOCH cohort had more patients with higher Ann Arbor stage, ABC phenotype and BCL2/BCL6 expression. Rate of treatment completion and complete response rate at the end of treatment were similar in both groups. DA.R-EPOCH was associated with increased grade 3/4 neutropenia and need for transfusions during treatment. The median follow up was 13.3 months and 10.9 months for DA.R-EPOCH and R-CHOP group respectively. There was no significant difference in OS and PFS among high-risk DLBCL patients treated with DA.R-EPOCH compared to R-CHOP: 3-year estimates of PFS with DA.R-EPOCH vs. R-CHOP was 77% and 71% respectively, while the 3-year estimates of OS was 80% and 66% respectively. The hazard ratio (HR) for PFS was 0.79 (95% CI- 0.28-2.29, P=0.67) and OS was 0.86 (95% CI- 0.26-2.78, P- 0.80). (Figure 1) Low baseline serum albumin levels, ECOG performance status >/=2, elevated LDH and high IPI were associated with poor OS and PFS.
CONCLUSION
In our retrospective analysis, treatment of patients with high-risk DLBCL with DA.R-EPOCH resulted in similar clinical outcomes, but was associated with increased incidence of grade 3/4 neutropenia and need for transfusions during treatment when compared to those receiving R-CHOP regimen. Patients receiving DA.R-EPOCH regimen had more adverse features in terms of disease stage and phenotype. A prospective randomized comparison is warranted between these two regimens for high-risk DLBCL. Until such prospective studies show benefit in any sub-set of DLBCL, DA.R-EPOCH should be used with judicious clinical discretion.
Ailawadhi: Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pharmacyclics: Research Funding; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal